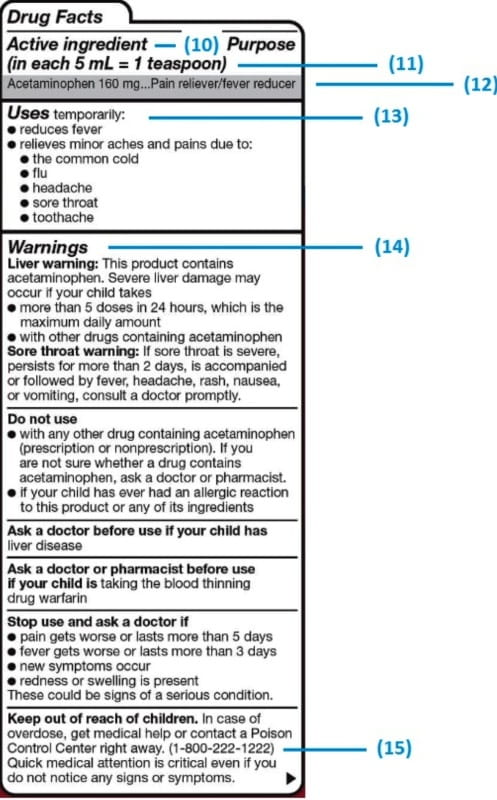
40 drug warning labels examples
Delay in Graphic Warning Labels on Cigarettes Cost Lives: Study - HealthDay The labels contain disturbing images and short messages about lesser-known risks of cigarette smoking, including stunted fetal growth, age-related macular degeneration, bladder cancer, cataracts, COPD, heart disease, strokes, head and neck cancer, type 2 diabetes and erectile dysfunction. Off-Label Prescribing in Pediatrics - Verywell Health The use of albuterol to treat children with asthma is a good example of the safe off-label use of a drug. Although commonly used in infants and toddlers, albuterol is only FDA-approved for use in children over 2 years old. Many other asthma inhalers, such as Dulera and Advair, are only FDA-approved for use in children over age 4 or 5.
10 black box warnings every physician should know | MDLinx Here, we've compiled a list of 10 black box warnings that every physician should know about when counseling patients on drug safety and treatment. Read on to learn more. 10 to look out for Aripiprazole (Abilify).

Drug warning labels examples
CBD Labeling Requirements and Guidelines - Avery FDA Sends Out Warnings One example of the warnings the FDA is sending to CBD manufacturers is a letter sent to The Dragon Tree Apothecary. The warning letter stated the CBD company is in violation for using false claims on their product labels and website. List of Black Box Warnings for Pharmacists | PharmaFactz For example: when atypical antipsychotics were assigned a black box warning for use in patients with dementia (as it increases the risk of death) - prescription use of antipsychotics for this population declined thereafter. The same is true for many other medicines, too. FDA Guidance on Medical Device Patient Labeling: Warnings and ... The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has published a guidance document dedicated to medical device patient labeling - a specific type of labeling intended to communicate important use- and safety-related information directly to the customers. The document provides additional clarifications regarding the ...
Drug warning labels examples. Drug Safety Communications | FDA Current Drug Safety Communications 6/30/2022 FDA warns about possible increased risk of death and serious side effects with cancer drug Copiktra (duvelisib) 6/1/2022 FDA approval of lymphoma... Look-Alike Drug Vials: Latest Stories & Gallery - Anesthesia Patient ... Ephedrine, Phenylephrine, Metoclopramide, Famotidine Cefazolin, Flumazenil Tranexamic Acid Injection (same medication labeled with multiple colors) Atropine Sulfate, Ondansetron, Dexmedetomidine (same medication labeled with multiple colors) Heparin Sodium Injection, Calcium Gluconate Submitted by Janette McVey, MD University of Missouri Healthcare High-Alert Medications: Definition & Examples - Study.com Common causes of such errors include poor communication, ambiguities in drug names, directions for use, medical abbreviations or writing, or patient misuse because of poor understanding of the ... Semaglutide: uses, dosage, side effects, brands - Drugs.com Warnings Call your doctor at once if you have signs of a thyroid tumor, such as swelling or a lump in your neck, trouble swallowing, a hoarse voice, or shortness of breath. You should not use semaglutide if you have multiple endocrine neoplasia type 2 (tumors in your glands), or a personal or family history of medullary thyroid cancer .
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration § 201.312 - Magnesium sulfate heptahydrate; label declaration on drug products. § 201.313 - Estradiol labeling. § 201.314 - Labeling of drug preparations containing salicylates. § 201.315 - Over-the-counter drugs for minor sore throats; suggested warning. § 201.316 - Drugs with thyroid hormone activity for human use; required warning. Drug Recalls, Withdrawals & Warnings (FDA Alerts) - Drugs.com B. Braun Medical Inc. Issues Voluntary Nationwide Recall of 0.9% Sodium Chloride for Injection USP 250ML in Excel Due to Fluid Leakage or Low Fill Volume. March 3, 2022 -- B. Braun Medical Inc. (B. Braun) is voluntarily recalling five (5) lots of 0.9% Sodium Chloride for Injection USP 250ML in Excel within the... March 3, 2022. Drug Alerts and Statements | FDA 1/28/2016 FDA warns consumers about health risks with Alikay Naturals - Bentonite Me Baby - Bentonite Clay 1/28/2016 FDA warns consumers not to use Viansilk's "Crema Piel De Seda" ("Silky Skin... Off-Label or Off-Limits: Should You Use a Drug for An Unapproved Use? Off-label drug use in inpatient cancer patients ranged from 18% to 41%, and 13% to 71% of adult patients received a minimum of one off-label chemotherapy drug. Patients with metastatic cancer and in need of palliative care received the most off label drugs. Off-label use that was not backed up by guidelines or drug compendia ranged from 7% to 31%.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Mar 29, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Subpart C - Labeling Requirements for Over-the-Counter Drugs. Sec. 201.66 Format and content requirements for over-the-counter (OTC) drug product labeling. Food Labeling Lawsuits | LegalMatch A food labeling lawsuit is a type of product liability claim. This type of claim typically involves warning defects. A warning defect involves a failure to warn public consumers of dangers or risks that are associated with the product. It may also involve general misinformation regarding the food product. A warning label lawsuit is a lawsuit ... How to Read a Package Insert | The Well Project For example, there is usually a recommendation against leaving the medicine out in temperatures over 30ºC (86ºF). So, if you pick up your prescription from the pharmacy on a hot day, do not leave it in the car while you run other errands! Patient Counseling Information How to Write a Warning Label to Avoid Product Liability For example, in the San Francisco Bay Area, warnings should be translated into Spanish, Tagalog, and Chinese, at least. Pictures might also be helpful, especially if assembly by the consumer is required and if assembly done improperly could create a risk of harm to the consumer.

Printable Prescription Warning Labels / How Well Does A Drug Work? Look Beyond The Fine Print ...
Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Improving Prescription Drug Warnings to Promote Patient Comprehension | JAMA Internal Medicine ...
Delay in Graphic Warning Labels on Cigarettes Cost Lives: Study The labels contain disturbing images and short messages about lesser-known risks of cigarette smoking, including stunted fetal growth, age-related macular degeneration, bladder cancer, cataracts,...
FDALabel: Full-Text Search of Drug Product Labeling | FDA For example, the product title search may result in some labeling that have the queried text appearing in the Highlights Limitation Statement, the Initial U.S. Approval, or the biosimilarity...
Pesticide Labels | US EPA Pesticide labels translate results of our extensive evaluations of pesticide products into conditions, directions and precautions that define parameters for use of a pesticide with the goal of ensuring protection of human health and the environment.
What are boxed warnings? - Drugs.com For example, Zyprexa Relprevv is a long-acting injectable antipsychotic medication that may be used to treat adults with schizophrenia. A serious reaction, called post-injection delirium sedation syndrome, can occur within three hours of Zyprexa Relprevv in less than 1 percent of people, and the drug carries a boxed warning about this syndrome.
Introduction to the New Prescription Drug Labeling by the FDA - Medscape Example of highlights section (for a fictitious drug). Limitations Statement. Highlights does not include all the information necessary touse a drug safely and effectively. For this reason, it is...
Common Drug Side Effects: Types & FDA Regulations - WebMD Another example is grapefruit juice, ... Sometimes these reports are numerous or serious enough for the FDA to take regulatory action, such as adding warnings to a drug's label.
Product Labeling Laws - Explained - The Business Professor, LLC Federal agencies heavily involved in product labeling laws include the CPSC, FTC, and FDA. Collectively, federal and state laws require manufacturers to place informative labels and warnings on various types of products based upon product category, materials or substance, and applicable safety standards. Several major laws are discussed below.
Black Box Warning - StatPearls - NCBI Bookshelf Boxed warnings (formerly known as Black Box Warnings) are the highest safety-related warning that medications can have assigned by the Food and Drug Administration. These warnings are intended to bring the consumer's attention to the major risks of the drug. Medications can have a boxed warning added, taken away, or updated throughout their tenure on the market. Over 400 different ...
What are Auxiliary Labels? - PTCB Test Prep Examples of common auxiliary labels include: Do not chew or crush Swallow whole Take with food or milk For rectal use only Shake well before use For external use only May cause drowsiness Protect from sunlight Take on an empty stomach Keep refrigerated For the eye (or ear) only May cause urine discoloration
FDA's Labeling Resources for Human Prescription Drugs | FDA Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
FDA Guidance on Medical Device Patient Labeling: Warnings and ... The Food and Drug Administration (FDA or the Agency), the US regulating authority in the sphere of healthcare products, has published a guidance document dedicated to medical device patient labeling - a specific type of labeling intended to communicate important use- and safety-related information directly to the customers. The document provides additional clarifications regarding the ...
List of Black Box Warnings for Pharmacists | PharmaFactz For example: when atypical antipsychotics were assigned a black box warning for use in patients with dementia (as it increases the risk of death) - prescription use of antipsychotics for this population declined thereafter. The same is true for many other medicines, too.
CBD Labeling Requirements and Guidelines - Avery FDA Sends Out Warnings One example of the warnings the FDA is sending to CBD manufacturers is a letter sent to The Dragon Tree Apothecary. The warning letter stated the CBD company is in violation for using false claims on their product labels and website.











Post a Comment for "40 drug warning labels examples"