44 eu language requirements for product labels
Language requirements for EU medical device labels The majority of member states, 21, require medical device labeling to be in their official language regardless of whether the device is intended for layman use or professional use. However, six member states will accept labeling provided in English as long as the device is for professional use only. In addition to Ireland and the United Kingdom ... European Language Translation Requirements for Medical Device Labeling ... The EU declaration of conformity shall, as a minimum, contain the information set out in Annex IV and shall be translated into an official Union language or languages required by the Member State (s) in which the device is made available." Shown below are the medical device language requirements for devices sold in European countries.
eu language requirements for product labels Call 24/7 (315) 601-7931 Westmoreland, NY 13490 Call 24/7 (315) 601-7931

Eu language requirements for product labels
EU product requirements | European Commission Safety requirements for goods in the EU market. Safety, labelling, packaging and marketing rules for products imported into the EU, technical standardisation and conformity rules, ecolabel rules, check what requirement your product needs. Import of goods in the EU - technical requirements. The role of customs in maintaining safety, health and ... EU MDR language requirements — what manufacturers and ... - Decomplix In the explanations on technical documentation in Annex II, there is also a list of the information that the manufacturer must include in the respective official languages, namely the label (s) on the product and its packaging (single unit packaging, sales packaging, transport packaging) as well as the instructions for use. GUI for medical software EU - Labeling/Marking Requirements - International Trade Administration Effective July 16, 2021, the EU required all CE marked products to have a label that identified a point of contact within the region. This requirement applies to products sold online and through traditional distribution channels.
Eu language requirements for product labels. EU: Language Requirements for Product Labels - GlobalTrade.net Belgium has three official languages: Dutch, French and German. All information which is required to appear on labels must be in the official language of the region where the product is commercialized (if necessary, further requirements for specific products may be obtained from the importer). eur-lex.europa.eu › legal-content › ENEUR-Lex - 52021PC0206 - EN - EUR-Lex - Europa Apr 21, 2021 · AI systems in the area of migration, asylum and border control management covered by this Regulation should comply with the relevant procedural requirements set by the Directive 2013/32/EU of the European Parliament and of the Council 49, the Regulation (EC) No 810/2009 of the European Parliament and of the Council 50 and other relevant ... Cosmetic product labelling and claims - CE.way EU COSMETICS LABELLING REQUIREMENTS. Just as attractive labelling is one of the most important features of the product when it comes to sales, so is correct labelling essential for the compliance of the cosmetic product with the cosmetics Regulation 1223/2009/EC and the UK Schedule 34 to the Product Safety and Metrology etc. (Amendment etc.) (EU Exit) Regulations 2019, as labelling is an ... EU Language Requirements | Obelis EU Language Requirements EU Language Requirements The label of a cosmetic product (container & outer packaging) should follow the language requirements which are exerted by the National Laws of the EU member states.
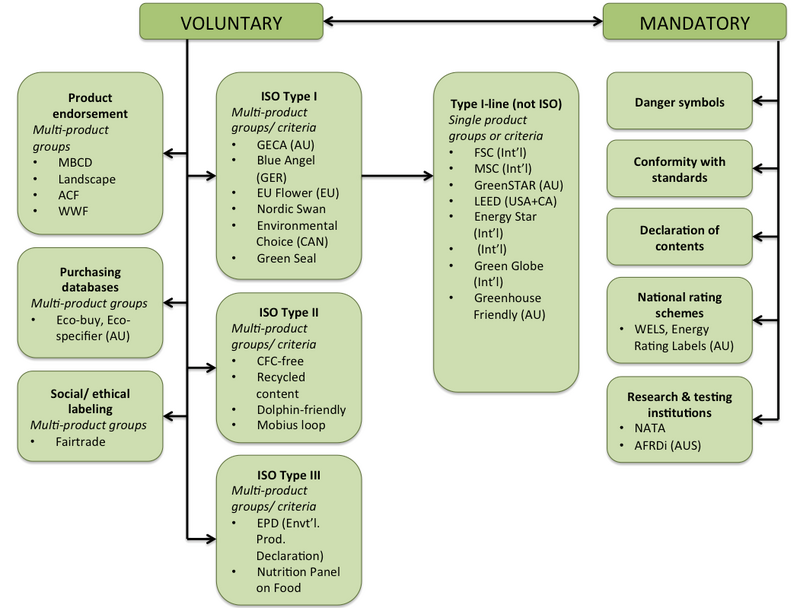
EU labels | European Commission EU Ecolabel- Products covered by the EU's Ecolabel initiative, criteria for establishing an Ecolabel, how to apply for an Ecolabel, application and annual fee rates. Energy labels. Energy efficient products - Requirements for energy efficient products, EU energy labelling and ecodesign rules, the EU's energy star programme. Labelling and packaging | Access2Markets - Europa The information provided by labels must be easy to understand, easily visible, clearly legible and indelible and must appear in the official language (s) of the Member State where the product is marketed. However, the use of foreign terms or expressions easily understood by the purchaser may be allowed. List of applicable legislation EU MDR - Language requirements - omcmedical.com Requirement: In Article 10 General Obligations of EU MDR 2017/745 states that the manufacturer should make the product information available in one or more Official languages of the member states where the product is put in place for use. The main goal about linguistic requirement is not to make complex process for the manufacturers but for the ... eur-lex.europa.eu › legal-content › ENEUR-Lex - 32020R0852 - EN - EUR-Lex - Europa In that regard, the Commission should take into account relevant Union law, including Directives 2001/42/EC (59), 2011/92/EU (60), 2014/23/EU (61), 2014/24/EU (62) and 2014/25/EU (63) of the European Parliament and of the Council, standards and current methodology, as well as the work of international organisations, such as the OECD. In that ...
eur-lex.europa.eu › legal-content › ENEUR-Lex - 32011R1169 - EN - EUR-Lex - Europa (4) According to Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (3) it is a general principle of food law to provide a basis for consumers to make informed choices in relation to food ... EU - Labelling Requirements | CE Intelligence The use of language on labels has been the subject of a Commission Communication, which points out that labelling of foodstuffs for sale to the final consumer must be in an easily understandable language which is generally interpreted to mean the language of the country of marketing (European Commission ,2010). europa.eu › youreurope › businessIdentifying product requirements - Your Europe Where no EU-wide rules exist, different specifications might apply in different EU countries. In such cases, you must only comply with the rules valid in your EU country. What are product requirements? EU law sets essential requirements to ensure products traded in the EU meet high health, safety, and environmental standards. eur-lex.europa.eu › legal-content › ENL_2017117EN.01017601.xml - Europa (1) Directive 98/79/EC of the European Parliament and of the Council (3) constitutes the Union regulatory framework for in vitro diagnostic medical devices. However, a fundamental revision of that Directive is needed to establish a robust, transparent, predictable and sustainable regulatory framework for in vitro diagnostic medical devices which ensures a high level of safety and health whilst ...
A Brief Reminder of the Language Requirements - Biorius Minimal legal requirements imposed by the European Cosmetics Regulation. According to Article 19 §5 of the EU Cosmetics Regulation, distributors have to ensure that a certain number of labeling requirements are properly translated in the national language(s) of the countries where the products are intended to be sold. These labeling ...
EOF
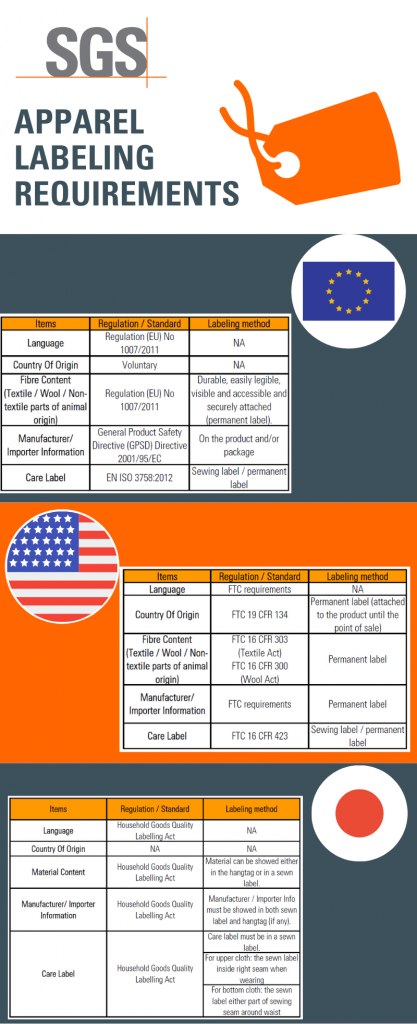
European Union Product Labelling Requirements: A Complete Guide EU Textiles Labelling Clothing and other products containing a minimum of 80% by weight of textile fibres must be labelled with the correct fibre composition (e.g. 100% Cotton or 100% Polyester). Further, the label must be permanent, which means it must either be attached to the clothing item or printed. A sticker is not enough. Product Examples
Product Labeling Requirements: What You Need To Know The US, Canada, Mexico, and the EU all require that your product packaging be written in local languages. In Canada, that means your labels need to be in English and French. In Mexico, that means Spanish. Choosing a Labeling Translation and Compliance Partner
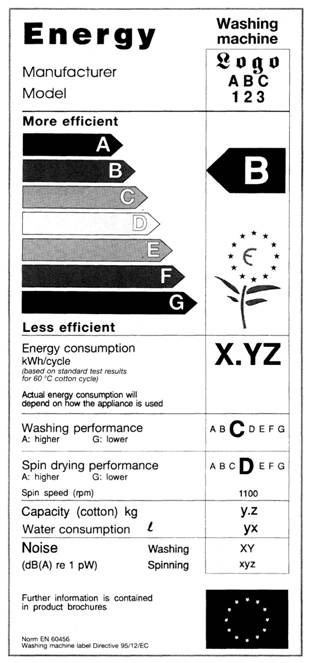
europa.eu › youreurope › businessEU energy labelling requirements - Your Europe Find more product-specific information on energy labelling and ecodesign requirements. Obligations of manufacturers and resellers If you are an EU-based manufacturer, an importer or an authorised representative of a non-EU manufacturer, you must do the following before placing a product on the EU market:
eur-lex.europa.eu › legal-content › ENEUR-Lex - 32011R1169 - EN - EUR-Lex - Europa (4) According to Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety (3) it is a general principle of food law to provide a basis for consumers to make informed choices in relation to food ...
Language Requirements For Eu Medical Device Labels European Language Translation Requirements for Medical Device … 6 hours ago The EU declaration of conformity shall, as a minimum, contain the information set out in Annex IV and shall be translated into an official Union language or languages required by the Member State (s) in which the device is made available.". Shown below are the medical device language requirements for devices sold ...
Product-information requirements | European Medicines Agency EMA's guidance explains the content that should be included in these documents, as well as standard headings and the most commonly used standard statements and terms in all official European Union (EU) languages plus Icelandic and Norwegian, and defines the format and layout for the product information. EMA's guidance is without prejudice to:
European Union - Labeling/Marking Requirements (part 1) EU - export MANDATORY MARKS AND LABELS CE Marking This is probably the most widely used and recognized marking required by the EU. Found in all "New Approach" legislation with a few exceptions, the CE marking demonstrates that a product meets all essential requirements (typically related to safety, health, energy efficiency and/or environmental concerns).
EU - Labeling/Marking Requirements - International Trade Administration Effective July 16, 2021, the EU required all CE marked products to have a label that identified a point of contact within the region. This requirement applies to products sold online and through traditional distribution channels.
EU MDR language requirements — what manufacturers and ... - Decomplix In the explanations on technical documentation in Annex II, there is also a list of the information that the manufacturer must include in the respective official languages, namely the label (s) on the product and its packaging (single unit packaging, sales packaging, transport packaging) as well as the instructions for use. GUI for medical software
EU product requirements | European Commission Safety requirements for goods in the EU market. Safety, labelling, packaging and marketing rules for products imported into the EU, technical standardisation and conformity rules, ecolabel rules, check what requirement your product needs. Import of goods in the EU - technical requirements. The role of customs in maintaining safety, health and ...























Post a Comment for "44 eu language requirements for product labels"